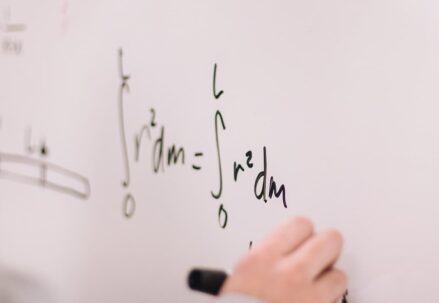Ludwig Boltzmann, a prominent name in theoretical physics during the late 1800s, was known for his steadfast belief in atomism. His revolutionary ideas helped shape the critical field of statistical mechanics. Boltzmann’s contributions to physics didn’t stop there. He also provided statistical interpretations of the second law of thermodynamics, ultimately enhancing our understanding of the universe’s physical laws.
Ludwig Boltzmann: A Champion of Atomism
During a time when the existence of atoms was still a hotly debated topic, Boltzmann stood firm in his belief. He was among the few elite physicists who drew a line in the sand, advocating for atomism. His stand was not just a matter of belief; his theoretical works provided convincing evidence for atomism. This crucial shift solidified atom’s place within the scientific community, making Boltzmann a significant figure in changing the course of science.
The Birth of Statistical Mechanics
Boltzmann’s groundbreaking work in developing statistical mechanics set a foundation for modern physics. This new field of study offered a bridge between the microscopic world of atoms and the macroscopic phenomena we observe. By integrating statistics and mechanics, Boltzmann’s innovative approach provided a powerful new perspective to understand physical systems.
Unraveling the Second Law of Thermodynamics and Kinetic Theory of Gases
Boltzmann also made substantial contributions to the kinetic theory of gases. His work helped establish a statistical interpretation of the second law of thermodynamics. Boltzmann’s insightful explanation tied together the microscopic behavior of individual atoms and molecules to the macroscopic, collective behavior of gas.
- This theory laid the groundwork for understanding how the random movement of gas particles leads to observable phenomena, such as pressure and temperature;
- Boltzmann’s statistical interpretation also shed light on the role of entropy in physical processes, a critical concept in thermodynamics.
The Formative Years: Ludwig Boltzmann’s Early Life and Education
On a winter day of February 20, in the year 1844, in Vienna, a young intellectual powerhouse was born into the family of the Boltzmanns. Named Ludwig, he was the son of Ludwig Georg Boltzmann, a dedicated tax official, and his wife, Ludwig Eduard Boltzmann.
In his early years, Ludwig was homeschooled by a private tutor, a common practice of the time. This period of homeschooling nurtured a sense of curiosity and independence in Ludwig, sparking his lifelong passion for knowledge.
As he matured, his education was further enriched at a local gymnasium, where he swiftly presented a remarkable talent for scientific subjects, most notably mathematics. Undoubtedly, the seeds of his future contributions to theoretical physics were sown in these formative years.
Upon turning 19, young Ludwig sought out higher education at the prestigious University of Vienna. In this academic setting, his curiosity thrived and he plunged into an in-depth exploration of mathematics and physics, his childhood passions. A mere three years later, Ludwig earned his doctorate, a testament to his academic prowess and tenacity.
His Ph.D. thesis was not a run-of-the-mill academic exercise. Instead, it revolved around the kinetic theory of gases, a topic that was later to become a cornerstone of his lifelong scientific contributions. This early foray into the kinetic theory of gases marked the commencement of Ludwig Boltzmann’s journey into theoretical physics—a journey that was to leave an indelible impression on the field.
Unveiling Boltzmann’s Pioneering Scientific Pursuits
Boltzmann’s Impactful Contributions to Physics
Ludwig Boltzmann’s name is etched in the annals of physics for his ground-breaking contributions. One of his early significant advances was expanding the concept of Maxwell’s distribution of velocities and energies for gaseous atoms. Boltzmann built upon James Clerk Maxwell’s seminal work, enriching our understanding of atomic behavior under varying conditions.
In the early 1870s, Boltzmann took strides in clarifying the second law of thermodynamics, grounding his explanations in atomic theory. His deep dive into the realm of atoms and their behaviors led to the formulation of the renowned Boltzmann entropy formula, a significant milestone in the field of statistical mechanics. This formula saw further refinement at the hands of Max Planck at the turn of the 20th century, culminating in its current form.
Boltzmann’s Visionary Blend of Mechanics and Probability
Boltzmann’s innovative approach to physics continued to shine as he sought to interpret the second law of thermodynamics. He ingeniously combined the principles of mechanics applied to atomic motion with the theory of probability—an innovative blend that added a new depth of understanding to this law.
Boltzmann didn’t stop at theory. He delved into detailed calculations within the realm of the kinetic theory of gasses, further solidifying his expertise and contributions in this field.
Boltzmann’s Recognition of the Brilliance of Maxwell’s Theory
In an era where the significance of James Clerk Maxwell’s electromagnetic theory was yet to be fully recognized, Boltzmann was among the first to grasp its profound importance. He dedicated extensive effort to exploring its depth, culminating in a two-volume treatise that stands as a testament to his scientific dedication and insight.
Boltzmann’s Endeavor in Black-Body Radiation and Quantum Mechanics
Boltzmann’s scientific curiosity was far-reaching. He delved into the derivation of black-body radiation based on Stefan’s law—a work later lauded as “a true pearl of theoretical physics” by Hendrik Antoon Lorentz, another stalwart in the field of physics.
In his visionary approach, Boltzmann extended his exploration to the quantum realm. He proposed the concept of discrete energy levels within a physical system, effectively making him one of the founding fathers of quantum mechanics.
Lighting the Spark: Boltzmann’s Esteemed Teaching Career
Boltzmann’s Induction into Academia
In 1869, Ludwig Boltzmann’s academic journey took a new and exciting turn when he secured his maiden professorship. The University of Graz, recognizing his exceptional prowess in mathematics and physics, appointed him as a professor of mathematical physics. This marked the beginning of Boltzmann’s impactful teaching career.
A Multidimensional Academic
During his lifetime, Boltzmann wore many academic hats and taught a spectrum of subjects across different universities. His academic repertoire spanned mathematics, experimental physics, and theoretical physics. While all these subjects were within his realm of expertise, it was theoretical physics that truly ignited his passion. And it showed in his lectures.
A Master Teacher
Boltzmann was not only revered as a legendary physicist but also celebrated for his teaching prowess. His students often spoke highly of his pedagogical style. Boltzmann had a knack for making complex scientific theories and mathematical equations come to life in his classes. His lectures weren’t just instructional; they were an experience.
Boltzmann was known to foster a learning environment filled with curiosity, creativity, and critical thinking. He crafted his lectures to be engaging and invigorating, feeding off his passion for theoretical physics and captivating his students with his enthusiasm.
A Student-Centered Pedagogue in a Teacher-Centric Era
In an era when professors in Austria and Germany were often authoritarian figures who maintained a strict teacher-student hierarchy, Boltzmann stood out as a striking exception. He adopted a student-centric approach to teaching, valuing his students’ voices and understanding.
Boltzmann believed in encouraging his students to think independently, ask questions, and challenge assumptions. This progressive teaching methodology was a rarity in his time, making his classes a sought-after destination for aspiring physicists.

Boltzmann’s Legacy: Impactful Theories in Modern Physics
The Far-Reaching Value of Kinetic Theory
Among Boltzmann’s numerous contributions, his work on the kinetic theory of gases stands as a shining beacon in the sphere of theoretical physics. This theory, in layman’s terms, is a powerful tool in studying dilute gases, where particles maintain an ample mean free path length and where collisions are far and few.
It offers a comprehensive understanding of the behavior of gases in varying conditions, proving instrumental in contexts well beyond Earth’s atmosphere. For instance, it finds applications in the celestial realm, helping astrophysicists decipher the behavior of stellar clusters and interstellar gases.
Additionally, the kinetic theory of gases also plays a pivotal role in understanding non-relativistic plasmas, a key aspect in the study of astrophysics and nuclear physics.
Boltzmann’s Monumental Contribution to Statistical Mechanics
Boltzmann’s embodiment of the second law of thermodynamics in a statistical manner birthed the foundation of statistical mechanics. This revolutionary theory revealed a fundamental link between the macroscopic and microscopic properties in any given system.
In the heart of this theory lies Boltzmann’s entropy formula, a principle that encapsulates the essence of a system in thermodynamic equilibrium. His entropy formula bridged the gap between statistical mechanics and thermodynamics, connecting two critical branches of physics in an unprecedented way.
In doing so, Boltzmann’s entropy formula gifted the scientific community with a tool to comprehend the flow of energy within a system at both macro and micro scales. This novel interpretation ushered in a new way of understanding and studying complex physical systems.
Navigating Through Shadows: Boltzmann’s Struggles and Demise
Facing Skepticism and Disbelief
Ludwig Boltzmann, despite his prodigious contributions to physics, faced a barrage of criticism and skepticism from his contemporaries. His uncompromising belief in atomism and the tangible reality of atoms put him at odds with influential thinkers of his time, such as Ernst Mach and Wilhelm Ostwald.
These renowned scholars and others rejected Boltzmann’s conceptualization of atoms and his statistical approach to understanding their behavior. Rather than embracing his innovative perspectives, they dismissed them, attacking Boltzmann’s work and leaving him to grapple with mounting scrutiny and the fear of his life’s work crumbling into irrelevance.
Battling the Beast of Despair
As Boltzmann withstood this onslaught of criticism, he was simultaneously wrestling with his internal demons, specifically the beast of depression. The constant critical onslaught and the weight of his scientific struggles ignited a fire of despair in him, causing his mental health to deteriorate.
This cauldron of professional criticism and personal anguish proved to be a lethal cocktail for Boltzmann. The man who had devoted his life to understanding the mysteries of the universe was enveloped by a profound sense of helplessness and desolation, with no escape in sight.
A Genius Lost Too Soon
In the grips of despair and seesawing between bouts of depression, Boltzmann succumbed to his internal demons. In a devastating stroke of fate, on September 5, 1906, while on vacation with his wife and daughter, Boltzmann ended his life. The world lost a brilliant physicist and a profound thinker in his tragic suicide.
Ironically, just weeks after his demise, experimental verification of Boltzmann’s work emerged, cementing his legacy as a trailblazer in atomism and statistical mechanics—a bitter testament to the often-treacherous journey of scientific discovery and acceptance.
Jacobi Mathematician: A Parallel Pursuit of Knowledge
While Ludwig Boltzmann’s contributions to physics are well-documented, it’s worth noting that he was not the only luminary of his time engaged in groundbreaking research. Carl Gustav Jacob Jacobi, a mathematician of great acclaim, conducted parallel pursuits of knowledge. Jacobi’s work in various mathematical fields, including elliptic functions, number theory, and differential equations, significantly advanced the realm of pure mathematics during the 19th century.
As Boltzmann delved into the mysteries of atoms and statistical mechanics, Jacobi explored the elegant intricacies of mathematical structures. Their parallel pursuits in the world of science and mathematics symbolize the diverse and profound avenues of human intellectual endeavor during the 19th century.
Conclusion
Today, Boltzmann’s final resting place is a poignant reminder of this great mind’s journey. His tombstone, inscribed with his iconic entropy formula, pays homage to his undeniable genius, his unwavering commitment to the validity of atoms, and his enduring contributions to physics.
Boltzmann’s story serves as a stark lesson in the perils that visionaries often face—skepticism, rejection, and solitude. Yet, his legacy reminds us that the pursuit of knowledge is rife with trials but equally abundant in rewards. The true value of scientific exploration lies not in immediate acceptance but in the lasting impact of ideas and the relentless quest for understanding the cosmos.